Study of ice nucleation on surfaces: the effect of surfaces on heterogeneous nucleation and ice growth at ambient conditions. (AV) See the project in slides
Ice is central to climate, geology and life. Understanding its behavior is essential for predicting the future of our planet. On average, 7% of the ocean’s surface is frozen; this alters ocean currents and limits the exchange of gases with seawater. Ice and snow coat 10% of the land permanently and up to half of the Northern Hemisphere in midwinter. Ice in clouds concentrate airborne chemicals and are sites were atmospheric chemistry takes place. Above the poles, clouds of ice grains host ozone-depleting reactions, forming holes in the stratospheric ozone layer at high latitudes that expose millions of people to increased ultraviolet radiation. Chemical reactions in snow on the ground can produce ozone and other environmental pollutants. Yet the molecular mechanisms underlying these processes remain largely unknown.
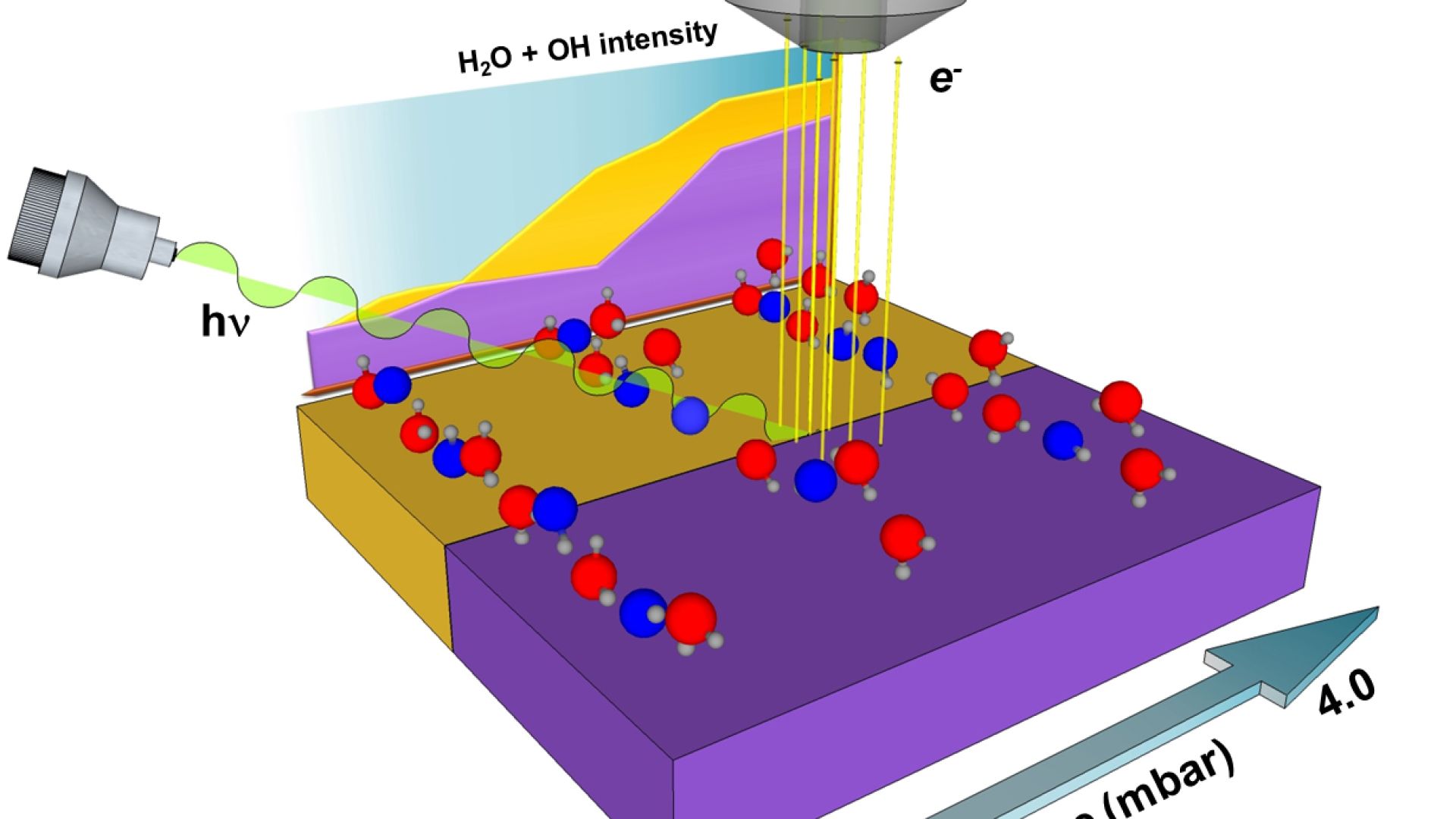
Without knowing more about ice formation, it is impossible to build snow or ice-cloud modules for atmospheric and climate models or to extrapolate laboratory studies to environmental conditions with enough confidence. A few years ago it was established the ten questions that science needs to study to gain this knowledge. The first question raised was to understand how ice nucleation occurs on solid surfaces. Ice often forms easily on solid surfaces through heterogeneous nucleation. This happens at higher temperatures than homogeneous nucleation (i.e. with pure water only) and it’s directly responsible of most of the rain. To understand why that happens, the molecular bases of the interaction of water molecules with such surfaces need to be studied. The goal of this research line is to study ice nucleation induced by surfaces to be able to obtain the knowledge needed for the modification of natural surfaces or the design of artificial surfaces with controlled ice nucleation properties.
Selected Recent Publications
Shimizu,; Maier, S; Verdaguer, A; Velasco-Velez, JJ; Salmeron, M
Progress Surf. Sci. 93, 87-107, 2018. DOI: 10.1016/j.progsurf.2018.09.004
“Substrate Dependence of the Freezing Dynamics of Supercooled Water Films: A High-Speed Optical Microscope Study”
Pach, E; Rodriguez, L; Verdaguer, A. Phys. Chem. B 122, 818-826, 2018. DOI: 10.1021/acs.jpcb.7b06933
“Revealing Water Films Structure from Force Reconstruction in Dynamic AFM”
Calo, A; Domingo, N; Santos, S; Verdaguer, A. Phys. Chem. C, 119, 8258-8265, 2015. DOI: 10.1021/acs.jpcc.5b02411
“Communication: Growing room temperature ice with graphene”
Verdaguer, A; Segura, JJ; Lopez-Mir, L; Sauthier, G; Fraxedas, J. Chem Phys., 138, 121101, 2013. DOI: 10.1063/1.4798941
Research Lines